제품특징
Advantages
- 질병 모델링 및 약물 발견에 이상적인 생리학적으로 관련된 3D 혈관 오가노이드 생성
- hPSC 유래 오가노이드의 효율적인 생성을 위한 최적화된 시약으로 표준화된 프로토콜 제공
- 약물 발견 또는 약물 테스트를 위한 96-well 형식의 높은 처리량 확장성 지원
General Workflow
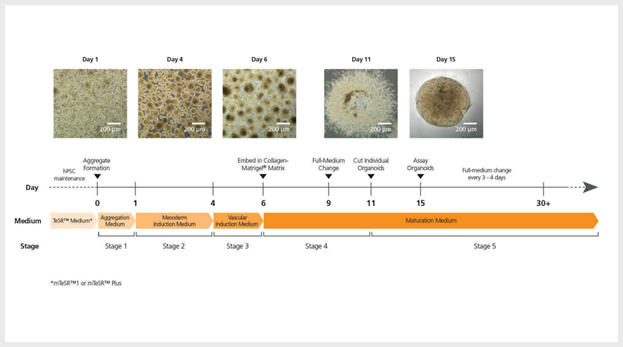
Figure 1. STEMdiff™ Blood Vessel Organoid Kit Protocol
mTeSRTM1-maintained hPSCs are seeded as single cells at 0.2 - 0.4 x 106 cells/well and mTeSR™ Plusmaintained hPSCs are seeded as single cells at 0.1 - 0.2 x 106 cells/well in 6-well ultra-low attachment plates in STEMdiff™ Blood Vessel Organoid Aggregation Medium, then incubated at 37°C for 1 or 2 days. Differentiation is then initiated by replacing the medium with STEMdiff™ Blood Vessel Organoid Mesoderm Medium and continuing incubation for 3 days. On day 4, vascular induction is initiated by replacing the medium with STEMdiff™ Blood Vessel Organoid Vascular Induction Medium, then incubating at 37°C for 2 days. Vascular aggregates are then embedded in a Matrigel®/collagen sandwich; aggregates sprout into vascular networks and mature into stable blood vessels when grown in STEMdiff™ Blood Vessel Organoid Maturation Medium for 5 days. Percentage of endothelial cells (CD31+ and CD144+) and pericytes (CD140b+) was evaluated via flow cytometry. Formation of lumen and 3D organization of endothelial cells and pericytes were analyzed using a confocal microscope.
혈관 네트워크 형성
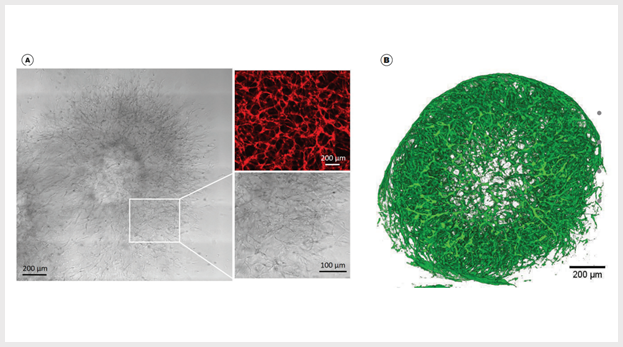
Figure 2. Vascular Aggregates Sprout Into Vascular Networks and Form Mature Blood Vessel Organoids in STEMdiff™ Blood Vessel Maturation Medium
(A) Vascular aggregates sprout into vascular networks after 5 days in STEMdiff™ Blood Vessel Maturation Medium and a Matrigel®/collagen sandwich. Vascular sproutings mature into blood vessel organoids when cultured in STEMdiff™ Blood Vessel Maturation Medium. Magnification: 10X; Insert: 25X; anti-human CD31 (red). (B) 3D reconstruction of z stack planes shows complex vasculature is formed after 22 days in STEMdiff™ Blood Vessel Maturation Medium and free-floating conditions. Magnification: 10X; anti-human CD31 (green).
혈관 마커의 발현
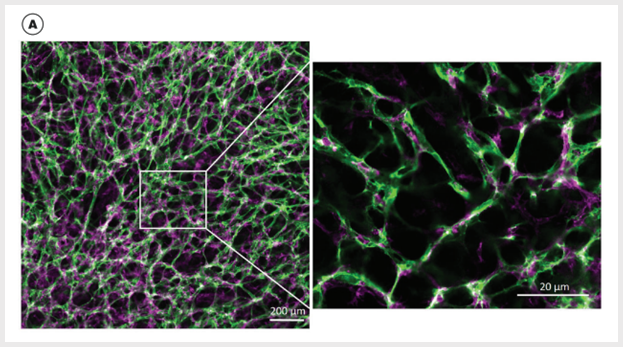
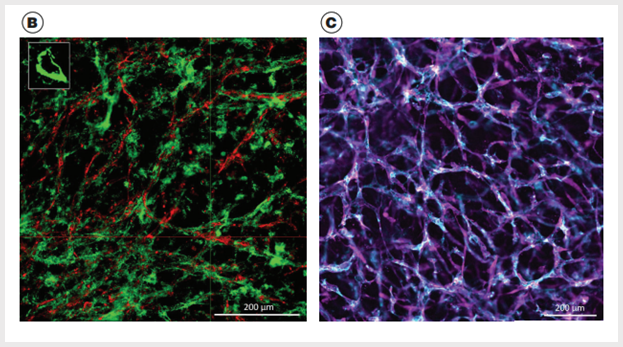
Figure 3. Vascular Networks Mature into Stable Blood Vessels When Cultured Within the Extracellular Matrix in STEMdiff™Blood Vessel Maturation Medium
(A) hPSC-derived blood vessel organoids are composed of hCD31+ cells (green) and hPDGFRβ+ cells (magenta); small quadrant shows tight endothelial and pericyte interactions. (B) hPSC-derived blood vessel organoids are composed of hCD31+ cells (red) and deposited collagen IV (green) (3D reconstruction of optical Z stacks); small quadrant shows blood vessel lumen. (C) hPSC-derived blood vessel organoids are composed of hCD31+ cells (blue) and alpha-smooth muscle actin cells (magenta).
혈관 구성의 중요한 세포 확인
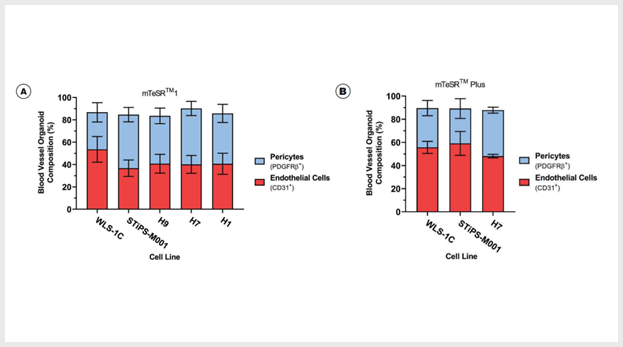
Figure 4. Blood Vessel Organoids Contain Both Endothelial Cells and Pericytes
(A) Induced pluripotent stem (iPS) and embryonic stem (ES) cell-derived blood vessel organoids contain endothelial cells (42.92 ± 3.97% CD31+, n=3 - 4) and pericytes (43.10 ± 3.31% CD140b+, n=3 - 4) in mTeSR™1-maintained cell lines WLS-1C (iPS), STiPS-M001 (iPS), H9 (ES), H7 (ES), and H1 (ES). (B) iPS and ES cell-derived blood vessel organoids contain endothelial cells (54.29 ± 3.72% CD31+, n=3) and pericytes (34.58 ± 3.49% CD140b+, n=3) in mTeSR™ Plus-maintained cell lines WLS-1C (iPS), STiPS-M001 (iPS), and H1 (ES).
Application


Figure 5. Deposition of Extracellular Matrix
(A) Diabetic conditions were applied to vascular networks, then collagen IV deposition was evaluated. Confocal microscopy revealed the formation of a complex network of tubes composed of CD31+ cells (red). Increased expression of collagen IV (green) was found in diabetic vascular networks compared to non-diabetic. Multiple small molecules were used in diabetic organoids to block signaling pathways involved in diabetic vasculopathies. Only addition of γ-secretase inhibitor DAPT and protein kinase activator (PKA) forskolin significantly reduced expansion of the collagen IV basement membrane. (B) The thickness of the collagen IV coat of individual vessels was measured in optical cross-sections (n=50 for non-diabetic, n=73 for diabetic (vehicle) & n=100 for diabetic (SB431542, Y-27632, CHIR99021, forskolin, and DAPT) blood vessel lumina from one independent experiment. Data are mean ± SD *P < 0.0001; two-tailed Student’s t-test). (C) The thickness of the collagen IV coat of individual vessels was measured in optical cross-sections (n=100 for non-diabetic & n=100 for diabetic blood vessel lumina from one independent experiment in two iPS cell lines & two ES cell lines. Data are mean ± SD *P < 0.0001; two-tailed Student’s t-test).
















 카트담기
카트담기 견적문의
견적문의